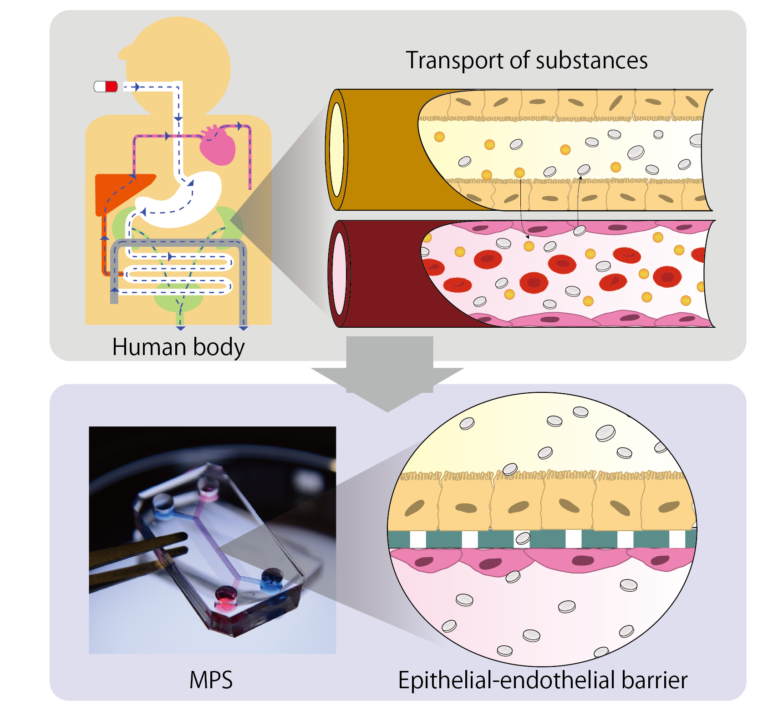
2D Culture: Epithelial endothelial barrier model
In normal physiology, an interface is formed between cells of organs and blood vessel cells and substances are transported through this interface. For example, in renal proximale tubules, the blood and urine are separated by an interface formed by vascular endothelial cells and the proximal tubule epithelial cells. Proteins, water and electrolytes are reabsorbed from urine side to blood side by transporters expressed in epithelial cells and drugs are excreted from blood to urine side.
Evaluating the transport of new drug through this interface and predicting their pharmacokinetics in vivo is extremely important for improving safety of new drugs. To predicts pharmacokinetic in vitro, it is essential to reproduce the interfaces using epithelial cells that has complete expression of transporters
In the case of the kidney, the expression of transporters in the conventional primary cells and immortalized cells was insufficient, making it difficult to predict drug excretion. On the other hands, by utilizing hiPS cell technology, one of Japan’s strength, it is possible to collect highly functional proximal tubule cells from kidney tissue created from hiPSCs. We have demonstrated that drug excretion can be evaluated by loading these cells into the MPS and reproducing the interface of the renal proximal tubules.
We aim to apply this technology to develop an MPS that will set new standard for pharmacokinetics and toxicity studies and we also continue to apply and develop this technology for other organs.
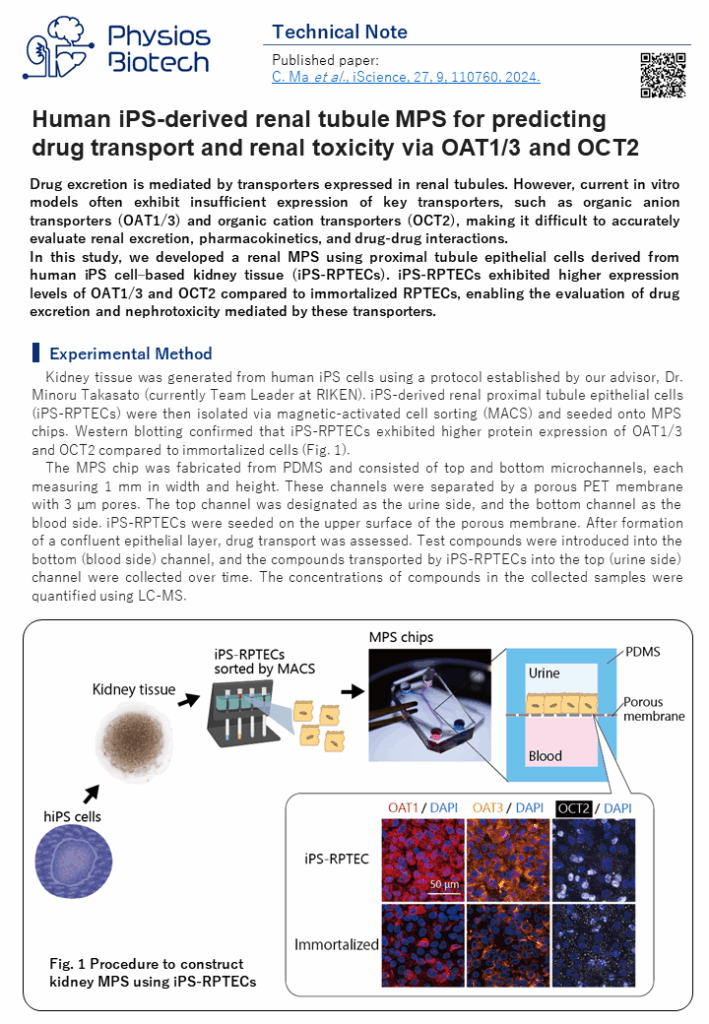
Technical note:Human iPS-derived renal tubule MPS for predicting drug transport and renal toxicity via OAT1/3 and OCT2
Drug excretion is mediated by transporters expressed in renal tubules. However, current in vitro models often exhibit insufficient expression of key transporters, such as organic anion transporters (OAT1/3) and organic cation transporters (OCT2), making it difficult to accurately evaluate renal excretion, pharmacokinetics, and drug-drug interactions.
In this study, we developed a renal MPS using proximal tubule epithelial cells derived from human iPS cell–based kidney tissue (iPS-RPTECs). iPS-RPTECs exhibited higher expression levels of OAT1/3 and OCT2 compared to immortalized RPTECs, enabling the evaluation of drug excretion and nephrotoxicity mediated by these transporters.
Reference: C. Ma et al., iScience, 27, 9, 110760, 2024.
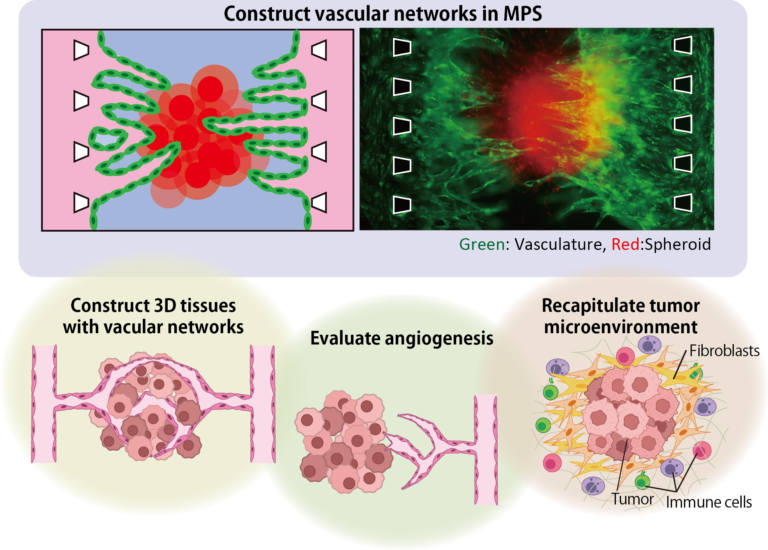
3D culture: Vascular network model
Blood vessels are important element in supplying oxygen and nutrients and discharging waste products. Blood vessels also maintain and improve function of organs through interaction with epithelial cells. however, 3D tissues, such as spheroids and organoids, created in the conventional dish method lacks blood vessels, making it difficult to reproduce the same function as in vivo.
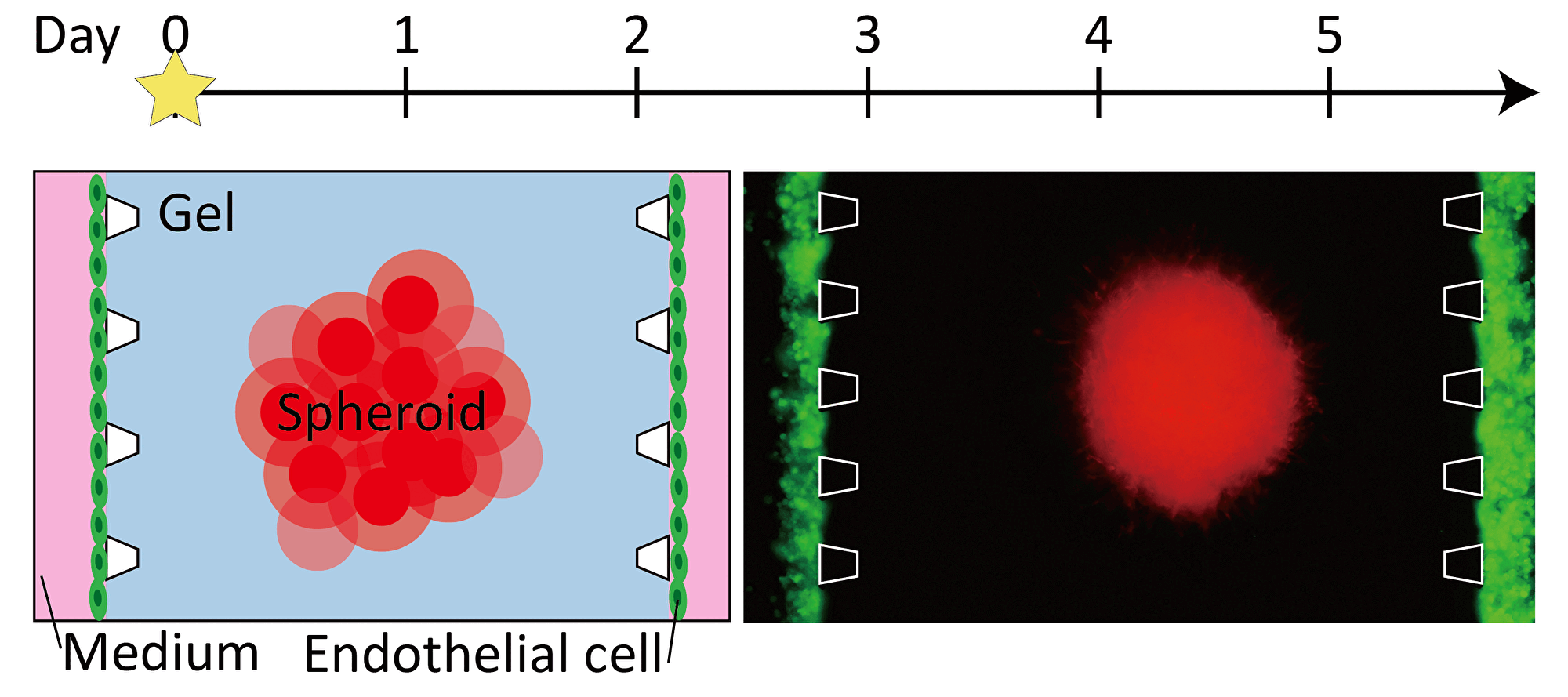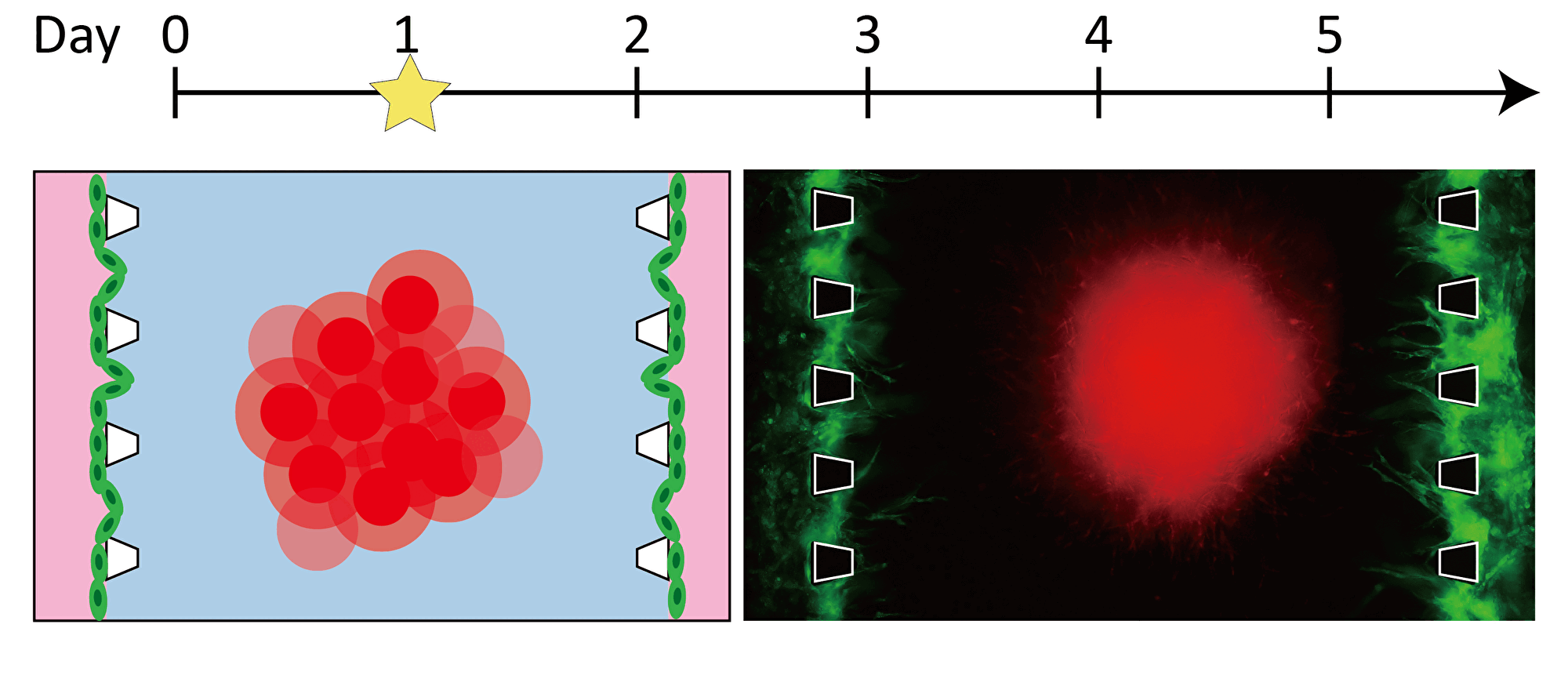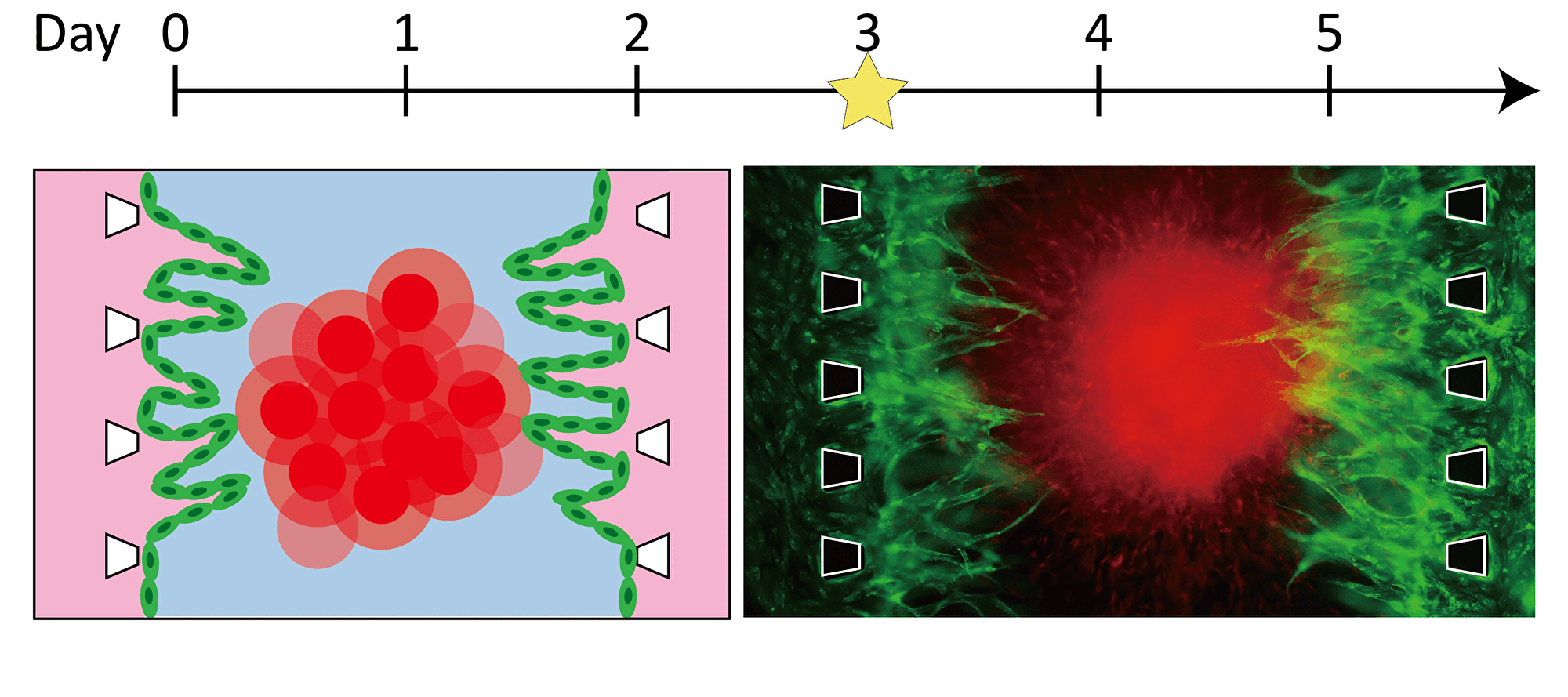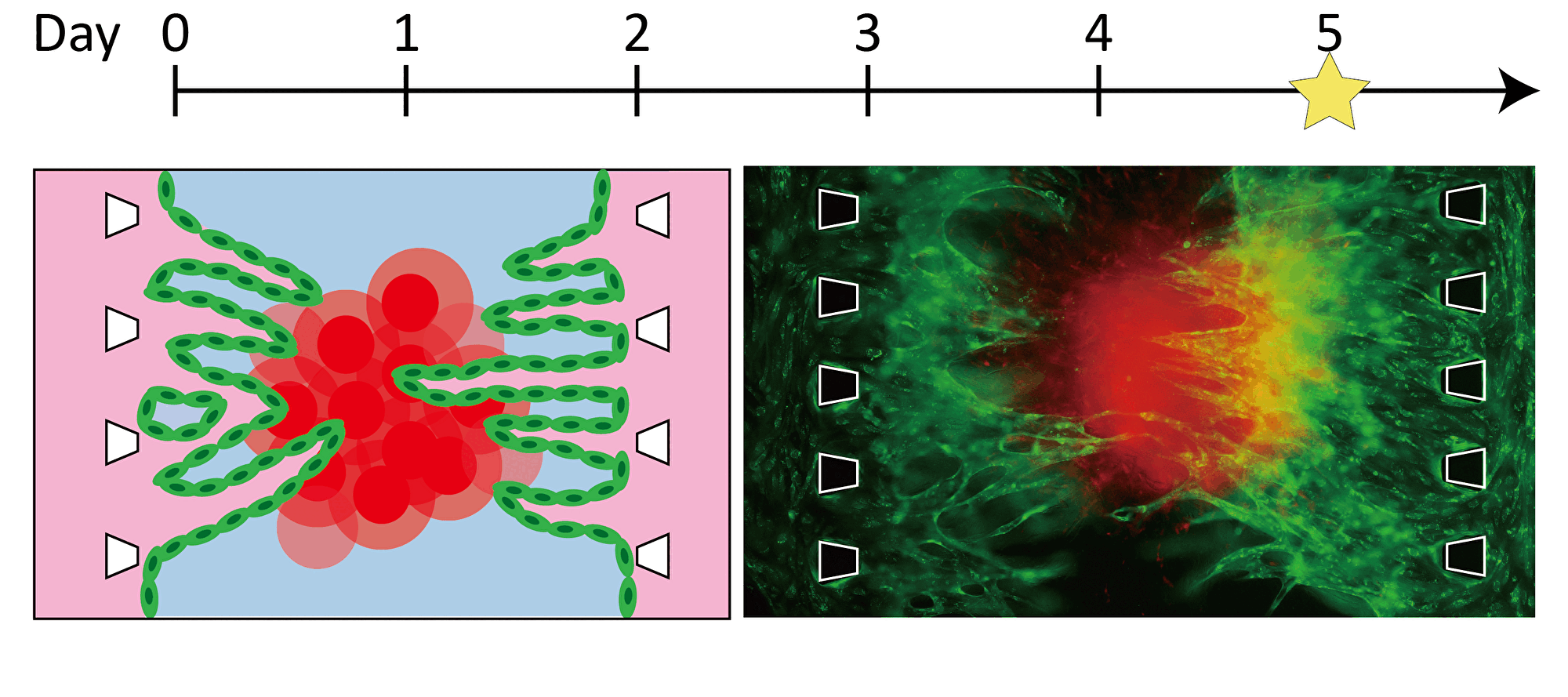
By inducing angiogenesis on MPS, it is possible to construct 3D tissue with connected blood vessels. A gel and 3D tissue are introduced into the central channel of three channel separated by pillars, and vascular endothelial cells are attached to the gel interface. Growth factors are secreted from the 3D tissue, and the newly formed blood vessels extend towards the 3D tissue according to the concentration gradient. The blood vessels formed in this way have a lumen, and it is possible to deliver culture medium and drugs to the 3D tissue through the blood vessels.
This MPS can also be applied to the field of cancer. The cancer microenvironment which includes tumor blood vessel and stromal cells, exists around the cancer, and this is the cause of the malignant transformation of the cancer and drug resistance. In addition, cancer actively induces angiogenesis to take in oxygen and nutrient and keeps growing. Our MPS makes it possible to reproduce the cancer microenvironment, evaluate angiogenesis which will be useful in developing cancer drugs that targets them.
We have cultured a wide variety of 3D tissues on MPS, including fibroblast spheroids, organoids etc. We will utilize this to develop assays that meet the customers needs.
Fundamental Technology: Construction of biological tissue with vascular network using angiogenesis
Progression of angiogenesis
 Original articles:
Original articles:
Y. Nashimoto et al., Integr. Biol., 9, 6, 506-518, 2017.
Y. Nashimoto et al., J. Vis. Exp., 134, e57242, 2018.
Visualization of flow in blood vessels.
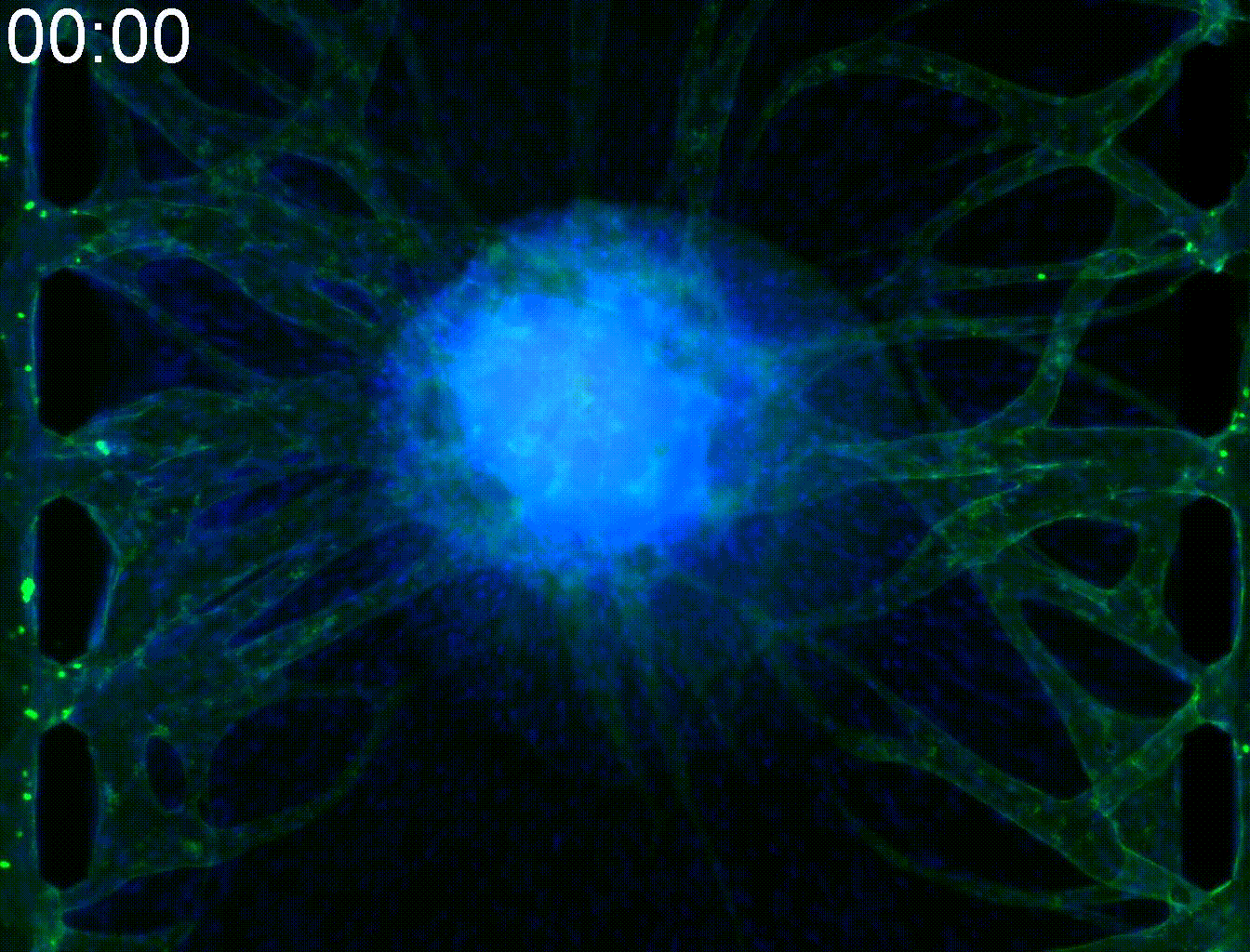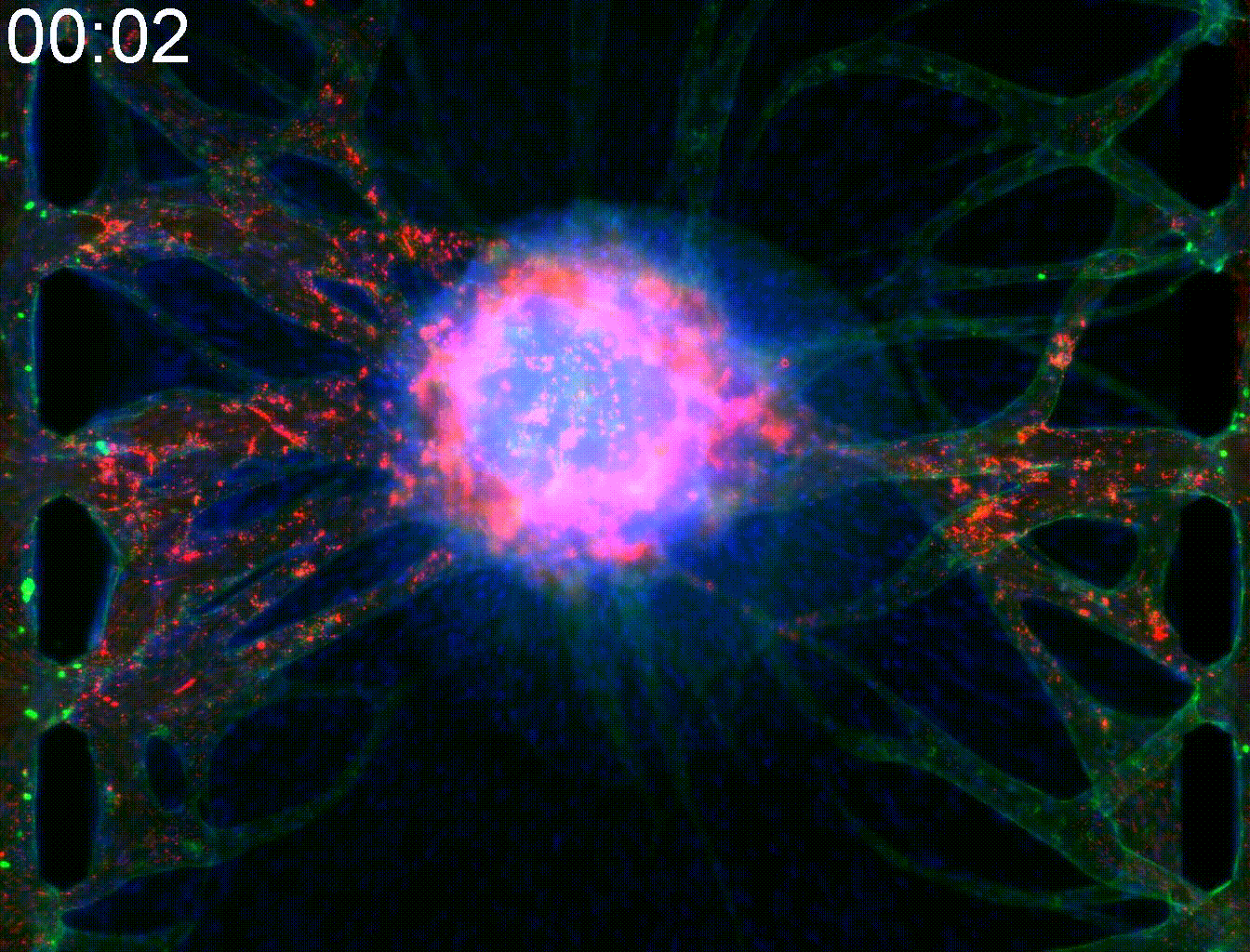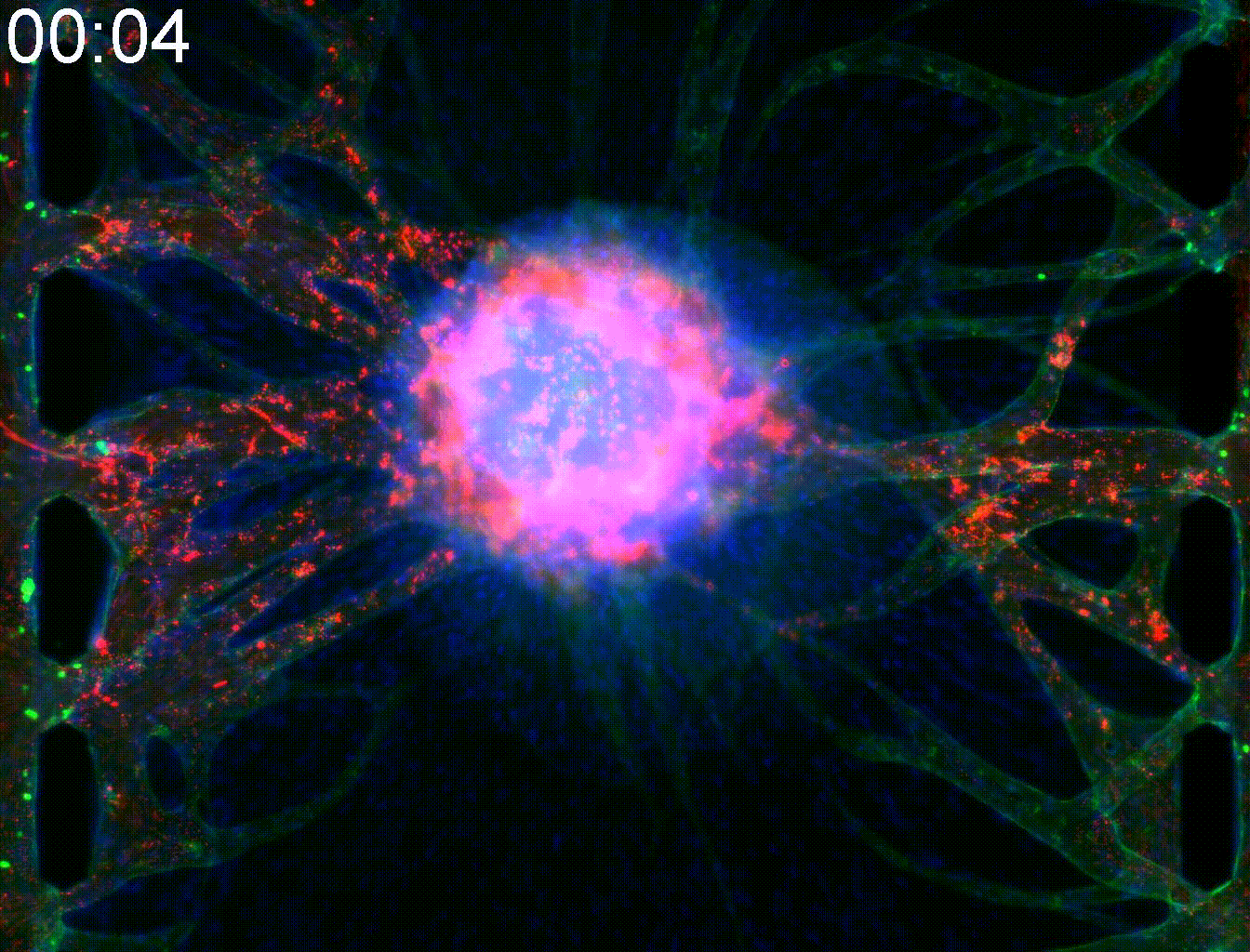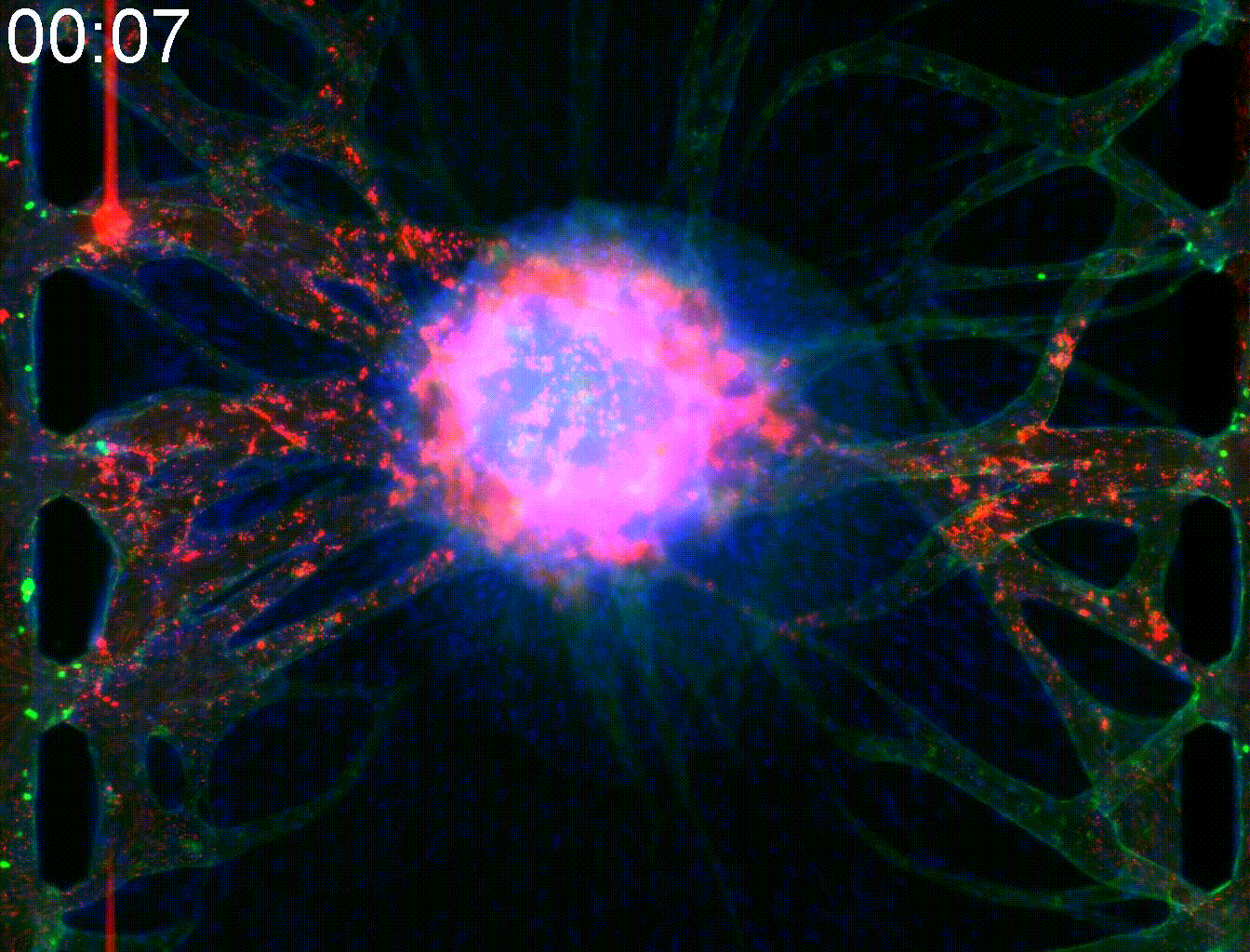
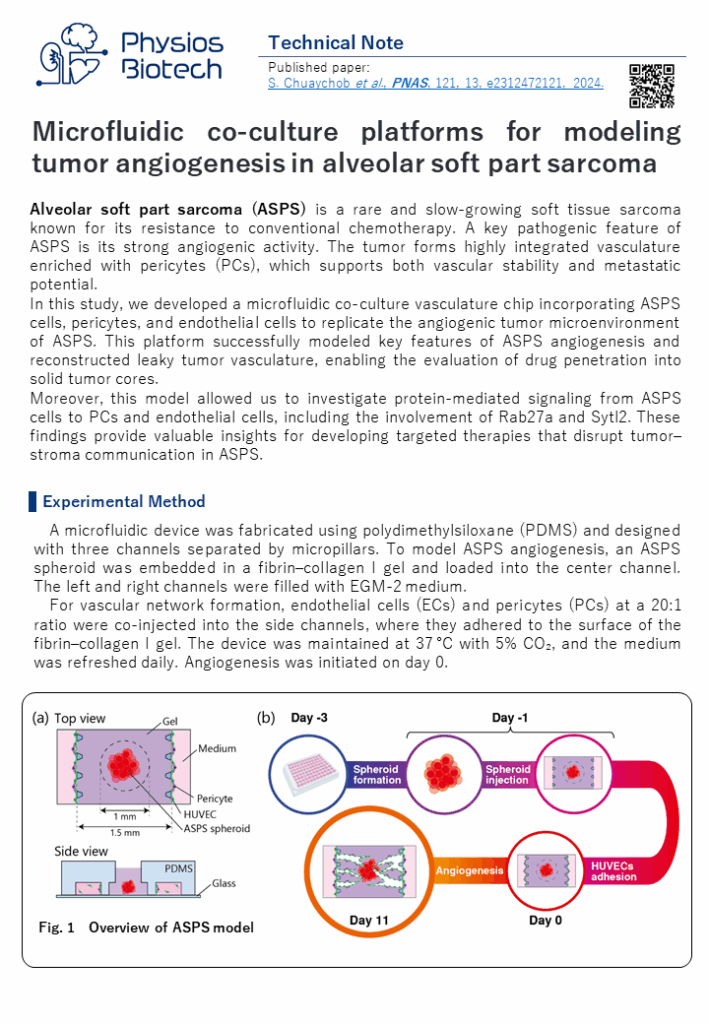
Technical note:Microfluidic co-culture platforms for modeling tumor angiogenesis in alveolar soft part sarcoma
Alveolar soft part sarcoma (ASPS) is a rare and slow-growing soft tissue sarcoma known for its resistance to conventional chemotherapy. A key pathogenic feature of ASPS is its strong angiogenic activity. The tumor forms highly integrated vasculature enriched with pericytes (PCs), which supports both vascular stability and metastatic potential.
In this study, we developed a microfluidic co-culture vasculature chip incorporating ASPS cells, pericytes, and endothelial cells to replicate the angiogenic tumor microenvironment of ASPS. This platform successfully modeled key features of ASPS angiogenesis and reconstructed leaky tumor vasculature, enabling the evaluation of drug penetration into solid tumor cores.
Moreover, this model allowed us to investigate protein-mediated signaling from ASPS cells to PCs and endothelial cells, including the involvement of Rab27a and Sytl2. These findings provide valuable insights for developing targeted therapies that disrupt tumor–stroma communication in ASPS.
Reference:S. Chuaychob et al., PNAS, 121, 13, e2312472121, 2024.